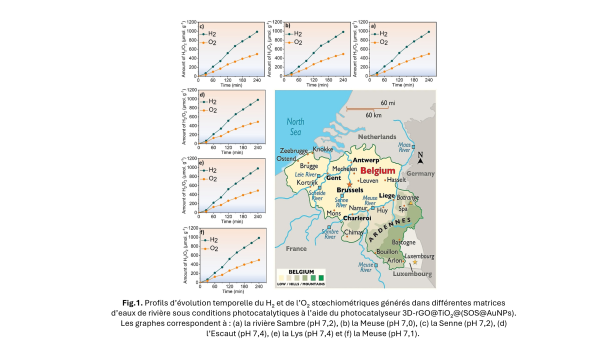
Producing "green" hydrogen from water from the Meuse River? It's now possible!
At UNamur, research is not confined to laboratories. From physics to political science, robotics, biodiversity, law, AI, and health, researchers collaborate daily with numerous stakeholders in society. The goal? Transform ideas into concrete solutions to address current challenges.